- Abemaciclib Intermediates
- Larotrectinib Intermediate
- Ivacaftor Intermediates
- AZD-9574 Intermediate
- AZD-5305 Intermediate
- Delafloxacin Intermediates
- Ponatinib Intermediates
- Baloxavir Intermediates
- Lisapram Intermediates
- Suzetrigine(VX548) Intermediate
- Resmetirol GL3196 Intermediate
- Dotinod Intermediates
- Other Intermediates
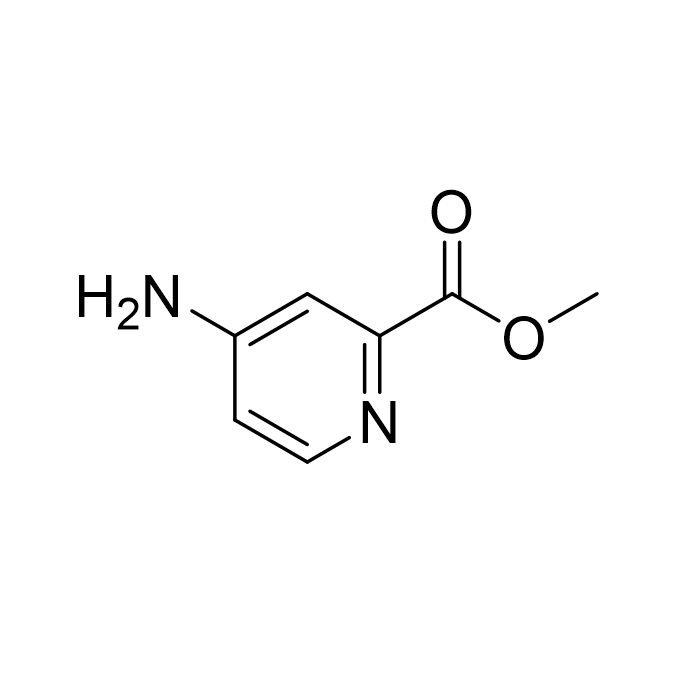
Methyl 4-aminopyridine-2-carboxylate CAS:71469-93-7
ZhonghanProduct Overview
4-Aminopyridine-2-carboxylic acid methyl ester is an important nitrogen-containing heterocyclic organic compound with a molecular formula of C7H8N2O2 and a molecular weight of about 152.15. From the chemical structure, it uses a pyridine ring as the parent nucleus, with an amino group (-NH2) connected to the 4th position of the pyridine ring and a methyl formate group (-C00CH3) connected to the 2nd position. This special structure gives the compound unique chemical properties and reactivity. In appearance, 4-aminopyridine-2-carboxylic acid methyl ester usually appears as a white to off-white crystalline powder with a certain stability and regular crystal morphology.
ZhonghanStructural formula
Molecular formula |
C7H8N2O2 |
Molecular weight |
152.15 |
Boiling point |
333.7±22.0 °C (Predicted) |
Density |
1.238±0.06 g/cm3 (Predicted) |
Storage conditions |
Keep in dark place, Sealed in dry, Room Temperature |
Acidity coefficient (pKa) |
4.35±0.10 (Predicted) |
Form |
Powder crystal |
Color |
White to light yellow to light orange |
ZhonghanPhysical properties
1. Chemical stability: At room temperature, pressure and general storage conditions, 4-aminopyridine-2-carboxylic acid methyl ester exhibits good chemical stability. However, since its molecules contain active amino and ester groups, reactions may occur in specific chemical environments. For example, the amino group has a certain alkalinity and is easily protonated under acidic conditions; the ester group may undergo hydrolysis in a strong acid-base environment to generate the corresponding carboxylic acid and alcohol. In addition, the electron cloud distribution on the pyridine ring will also affect the reactivity with other reagents.
2. Solubility: This product is slightly soluble in water. This is because although there are polar amino and ester groups in its molecular structure, the overall polarity of the molecule is relatively weak, and the interaction with water molecules is limited. However, it is soluble in a variety of organic solvents, such as ethanol, acetone, and dichloromethane. This solubility feature enables it to be fully dispersed and participate in the reaction in a suitable solvent system in organic synthesis reactions, providing convenience for subsequent synthetic operations.
3. Reactivity: The reactivity of 4-aminopyridine-2-carboxylic acid methyl ester mainly comes from its amino group and ester group. The amino group is nucleophilic and can react with a variety of electrophilic reagents, such as reacting with acyl halides and acid anhydrides to form corresponding amide derivatives, and reacting with aldehydes and ketones to form imines. The ester group can be hydrolyzed under alkaline or acidic conditions to generate 4-aminopyridine-2-carboxylic acid methyl ester, and can also undergo ester exchange reactions with alcohols to generate different lipid compounds. At the same time, the pyridine ring can also participate in some electrophilic substitution reactions and addition reactions, further expanding the application range of this compound.
ZhonghanApplication Areas
1. Pharmaceutical field: As an important pharmaceutical intermediate, 4-aminopyridine-2-carboxylic acid methyl ester has a wide range of applications in drug research. Nitrogen-containing heterocyclic compounds often play an important role in drug molecules and have good biological activity and pharmacokinetic properties. By structurally modifying and functionalizing 4-aminopyridine-2-carboxylic acid methyl ester, it is possible to synthesize a variety of drug factors with special biological activities, such as antibacterial drugs, antiviral drugs, antitumor drugs, etc. Its unique structure and reactivity provide rich possibilities for the design and synthesis of drug molecules.
2. Pesticide field: In the pesticide industry, 4-aminopyridine-2-carboxylic acid methyl ester also has certain applications. Nitrogen-containing heterocyclic pesticides have the advantages of high efficiency, low toxicity, and low residue, which meet the development needs of modern green agriculture. By utilizing the special structure and reactivity of this compound, pesticide varieties with good insecticidal, bactericidal, and herbicidal activities can be prepared, providing effective protection for agricultural production.
3. Materials Science: With the continuous development of materials science, methyl 4-aminopyridine-2-carboxylate has also emerged in the preparation of some new organic functional materials. For example, it can be used as a monomer to participate in polymerization reactions to prepare nitrogen-containing heterocyclic polymer materials with special properties. These materials have excellent electrical properties, thermal stability and mechanical properties, and can be used in the fields of electronics, aerospace, etc., such as the preparation of high-performance insulating materials, conductive polymers, etc.

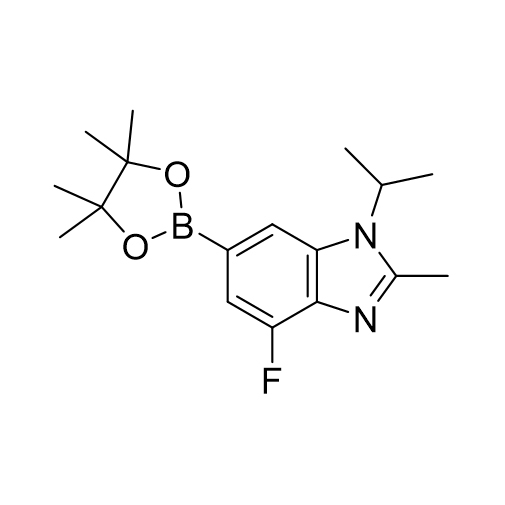
![6-Bromo-4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole CAS:1231930-33-8](/source/9e9faa51f944bc7ac934c145f661fec3/cas1231930-33-8.jpg)
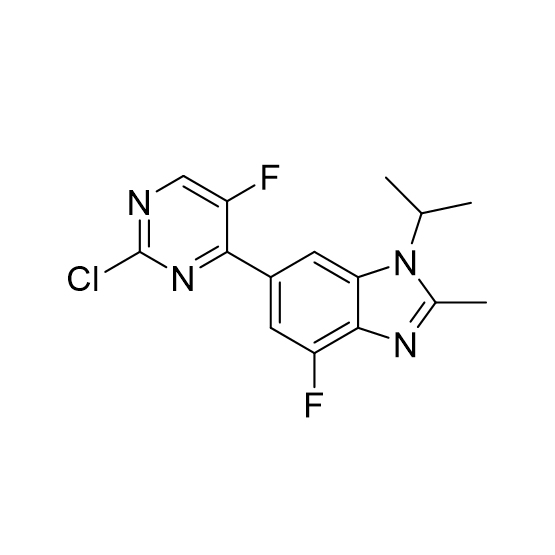
![5-Hydroxypyrazolo[1,5-a]pyrimidine CAS:29274-22-4](https://ecdn6.globalso.com/upload/p/3069/image_product/2025-02/cas29274-22-4.jpg)
![5-Chloropyrazolo[1,5-a]pyrimidine CAS:29274-24-6](/source/2d2e4c8649a6444c8114fbceac0e361d/cas29274-24-6.jpg)
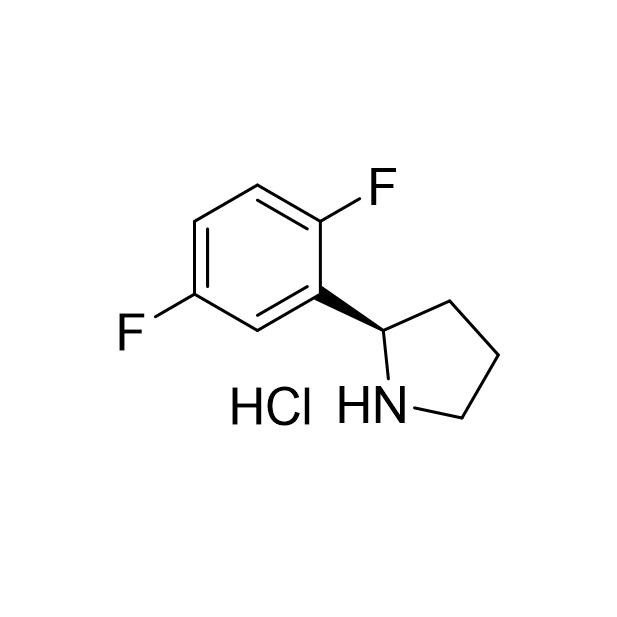
![5-Chloro-3-nitropyrazolo[1,5-a]pyriMidine CAS:1363380-51-1](/source/c735e97d9d2434baa533916048357d17/cas1363380-51-1.jpg)